Pipeline
Our product candidates are armed with one or more of our innovative enhancements. These include CAR constructs, technologies, and manufacturing protocols designed to give T cells more potent cancer cell killing capabilities.
Our product candidates are armed with one or more of our innovative enhancements. These include CAR constructs, technologies, and manufacturing protocols designed to give T cells more potent cancer cell killing capabilities.
Ronde-cel (rondecabtagene autoleucel, or LYL314) is a dual-targeting CD19/CD20 CAR T-cell product candidate designed to target B cells that express either CD19 or CD20, two antigens present on B-cell lymphoma, with full potency. Ronde-cel is manufactured with a process that generates CAR T cells with enhanced antitumor activity.
Lymphoma is a blood cancer that begins in lymphocytes and spreads primarily in lymph nodes, but can also metastasize to the liver, kidney, brain, and other organs. Lymphomas are classified as either Hodgkin or non‑Hodgkin lymphoma (NHL). NHL is the more common form of lymphoma, representing approximately 90% of all lymphomas. B-cell lymphoma is the most common type of NHL. Ronde-cel is initially in development for patients with aggressive relapsed and/or refractory large B-cell non‑Hodgkin lymphoma, more commonly referred to as large B-cell lymphoma (LBCL), including subtypes that represent approximately 35% of the more than 80,000 patients estimated to be diagnosed with NHL in the United States in 2025 and of the more than 545,000 patients worldwide.
Pivotal Trial Recruiting Now PiNACLE is a single-arm pivotal trial evaluating ronde-cel in patients with large B-cell lymphoma receiving treatment in the third- or later-line setting (disease has progressed on at least two prior lines of therapy). The trial is expected to enroll approximately 120 patients. Patients may be treated with ronde-cel in either the inpatient or outpatient setting. The primary endpoint of the trial is overall response rate.
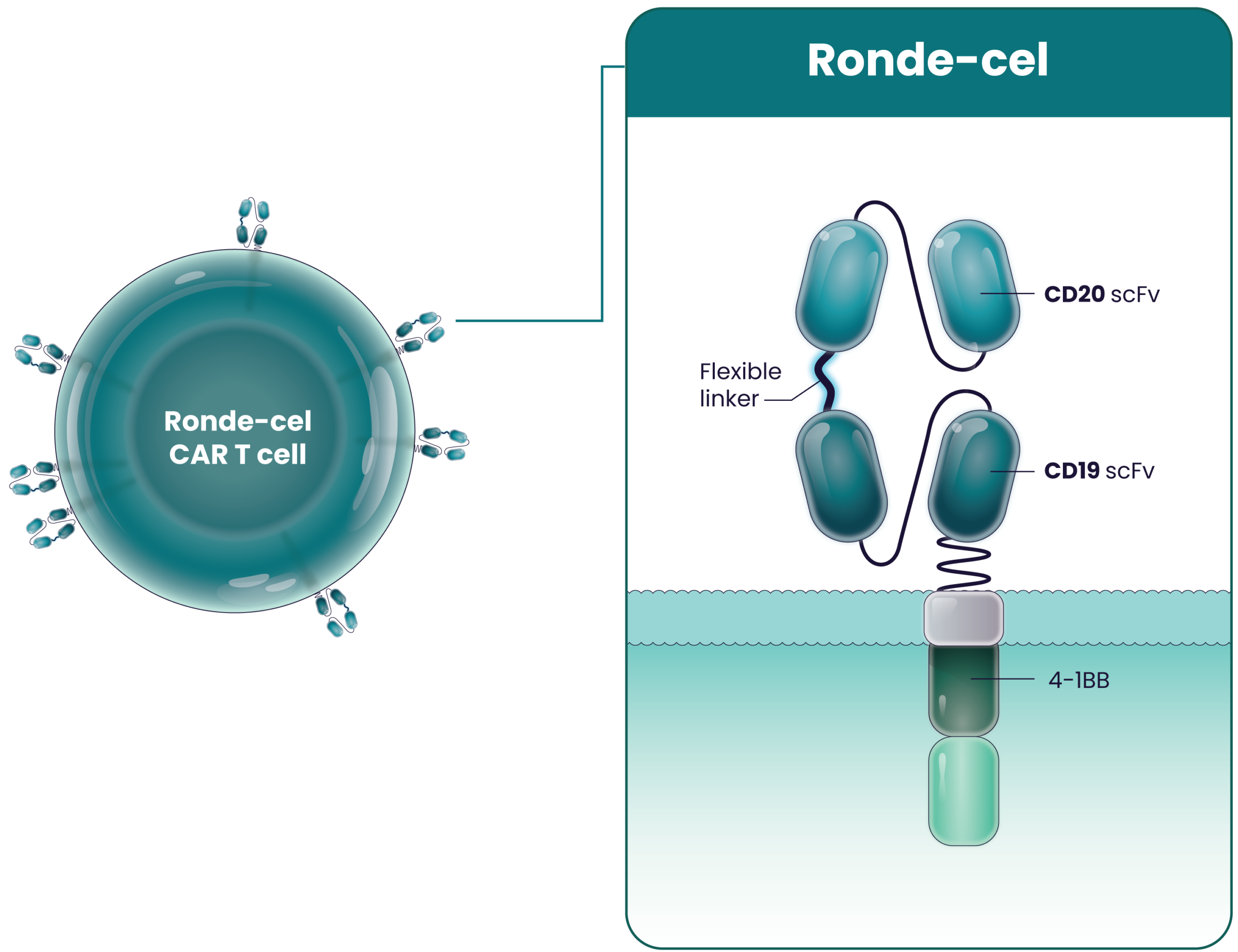
Ronde-cel (rondecabtagene autoleucel, or LYL314) is a dual-targeting CD19/CD20 CAR T-cell product candidate designed to target B cells that express either CD19 or CD20, two antigens present on B-cell lymphoma, with full potency. Ronde-cel is manufactured with a process that generates CAR T cells with enhanced antitumor activity.
Lymphoma is a blood cancer that begins in lymphocytes and spreads primarily in lymph nodes, but can also metastasize to the liver, kidney, brain, and other organs. Lymphomas are classified as either Hodgkin or non‑Hodgkin lymphoma (NHL). NHL is the more common form of lymphoma, representing approximately 90% of all lymphomas. B-cell lymphoma is the most common type of NHL. Ronde-cel is initially in development for patients with aggressive relapsed and/or refractory large B-cell non‑Hodgkin lymphoma, more commonly referred to as large B-cell lymphoma (LBCL), including subtypes that represent approximately 35% of the more than 80,000 patients estimated to be diagnosed with NHL in the United States in 2025 and of the more than 545,000 worldwide.
Pivotal Trial Recruiting Now The PiNACLE – H2H trial is a Phase 3 head-to-head CAR T-cell therapy randomized controlled trial evaluating ronde-cel versus Investigator’s choice of either lisocabtagene maraleucel, often referred to as liso-cel, or axicabtagene autoleucel, often referred to as axi-cel, in patients with relapsed or refractory (R/R) LBCL receiving treatment in the second-line setting (disease has progressed during or after first-line therapy). The trial is expected to enroll approximately 400 patients with R/R LBCL (200 per arm). The primary endpoint of the trial is event-free survival. Patients may be treated with ronde-cel in either the inpatient or outpatient setting.

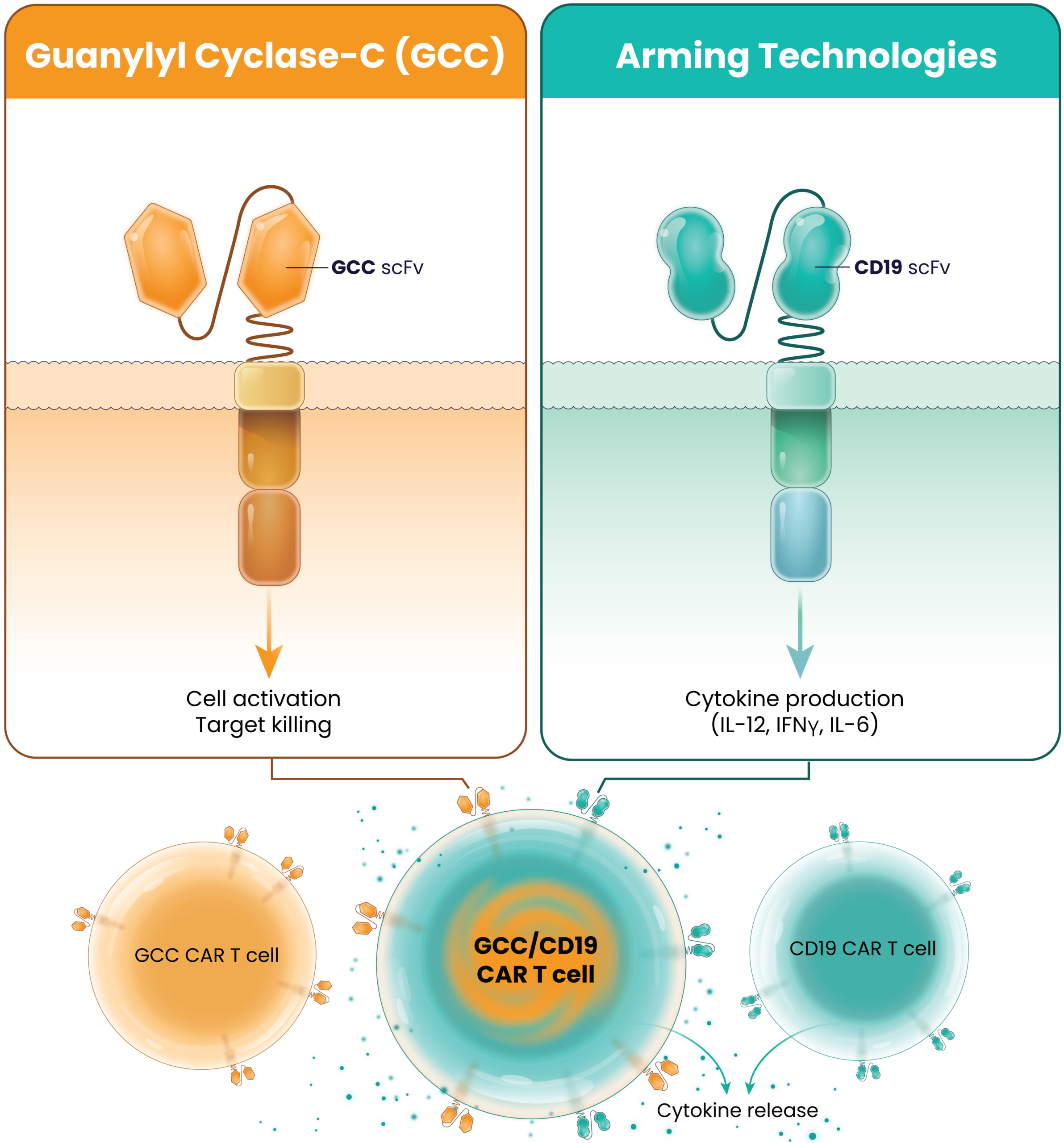
LYL273 is a novel autologous guanylyl cyclase-C (GCC)-targeted CAR T-cell product candidate for the treatment of metastatic colorectal cancer (mCRC) and other GCC-expressing cancers. GCC is a receptor that plays a key role in the regulation of intestinal electrolyte homeostasis. It is expressed on more than 95% of colorectal cancers and a majority of pancreatic adenocarcinomas. The expression of GCC in healthy tissue is limited to the gastrointestinal tract, where it is largely sequestered by tight junctions from the circulation. LYL273 is designed to improve CAR T-cell expansion, immune cell infiltration into the cancer, and cancer cell killing in the hostile solid tumor microenvironment. We believe these properties are needed to improve outcomes for patients with mCRC.
Colorectal cancer is the second leading cause of cancer deaths worldwide. Approximately 53,000 people were expected to die from CRC in the United States in 2025. The incidence of CRC is rising in people younger than 55 years old, and it is currently the deadliest cancer among young men and the second deadliest among young women.
Phase 1 A Phase 1 dose-escalation dose-expansion trial is ongoing for patients with relapsed or refractory metastatic colorectal cancer who have received at least two prior lines of therapy.